|
Intended Use |
Qualitative or quantitative detection of the ORF1ab and N genes of the SARS-CoV-2.
|
|
Specifications |
48 tests/kit |
|
Specimen type |
Serum/Plasma, Whole blood, Nasopharyngeal/ Oropharyngeal swab, Anal swab, Sputum, Tracheal Aspirate, Broncheo ,Alveolar Lavage (BAL) fluid, Pleural fluid and Urine. |
|
Sensitivity |
10 copies/PCR |
|
Precision |
Within / between batches CV < 5% |
|
Expiration Date |
The kit is best used within 12 months from manufacturing ,please stored at – 20 ± 5 ℃. |
|
Specificity |
No Cross-activity detected with other coronavirus (SARS, MERS, OC43, NL63, 229E and HKU1), influenza virus (H1N1, H3N2 and B), respiratory syncytial virus (RSV long strain), adenovirus, metapneumovirus, mycobacterium tuberculosis, bordetella pertussis, staphylococcus aureus, mycoplasma pneumoniae and chlamydia pneumoniae |
|
Instrument |
ABI 7500 Real-Time PCR Systems, ABI QuantStudioTM Dx Real-Time PCR Systems and ABI ViiA 7 Dx Real-Time PCR Systems and SLAN-96 Real-Time PCR Systems. |
Higher sensitivity, crack "false negative" (can detect asymptomatic people)
·All 27 cases of 2019-nCoV infection were confirmed;
·Among the 50 pharyngeal test specimens or nasopharyngeal swab samples suspected of 2019-nCoV infection, the detection rate increased by 33.3% compared with the approved marketed products (follow the table below);;
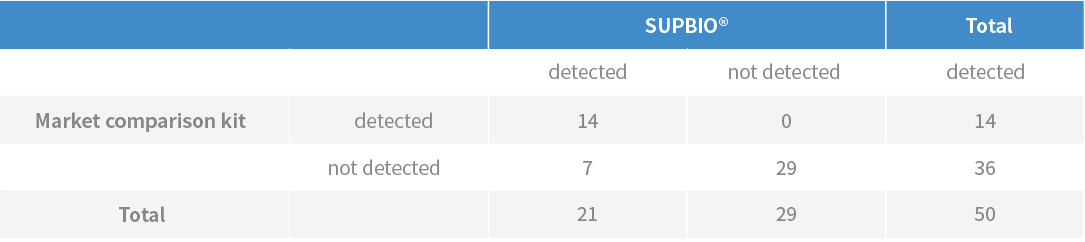
※ Seven more suspected patients detected by SUPBIO than other products, these patients were confirmed to be infected by the SARS-CoV-2.
High specificity
检测Detected 6000 healthy people and excluded patients with SARS-CoV-2 infection, all were not detected;
Quantitative detection
The kit contains quantitative reference products to provide quantitative indicators for scientific or clinical research;
Add internal standard and monitor the whole process
Add endogenous internal standard to monitor sample collection, nucleic acid extraction and amplification process to avoid false negative results [3];
Multi-scenario application
There are various types of testable specimens to meet the needs of a variety of clinical scenarios, increase the sampling of different parts of the same patient, reduce the "false negative" results [3]
A variety of nucleic acid extraction and PCR instrument can be applied to facilitate laboratories in various medical institutions to carry out new coronavirus detection projects;
It can be used for incubation crowds, returning to cities and returning to work, medical examinations for employees of enterprises, and screening for students returning to school, to stop the spread of the epidemic in time, and to help resume work and resume production.
reference
[1] WHO. Coronavirus disease (COVID-19) outbreak [EB/OL]. [2020-02-18]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
[2] 中华医学会检验医学分会. 新型冠状病毒肺炎病毒核酸检测专家共识 [J] . 中华医学杂志,2020,100( 00 ): E003-E003.
[3] 莫茜, 秦炜, 傅启华, 等. 正确认识新冠病毒核酸检测的影响因素 [J] . 中华检验医学杂志,2020,43( 00 ): E002-E002.